Technology informed by unparalleled Decentralized Trials (DCT) operational experience and delivered at global scale.
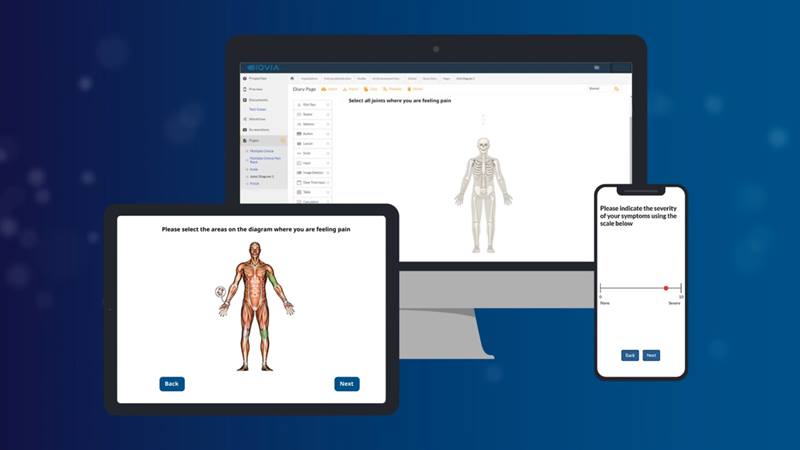





























Assessments run on devices patients already know and use. Collect clean, reliable, direct-from-patient data that is ready to use in real time across your clinical workflows.
Explore how IQVIA eCOA can help you improve speed and agility with transformative technology to accelerate delivery of new treatments and vaccines to global markets faster.

Save valuable time at start up with pre-built and easy-to-modify assessments.
Get real-time patient data and insights sooner to improve decision making and accelerate deployment.
Drive efficiency and accelerate trials with autogenerated regulatory and project documentation.
Leverage the industries largest digital assessment library.
The Sculptor tool accommodates robust reporting and integration. It’s dynamic and fully customizable and can be modeled on multiple devices in real time.
Protocol changes can be made centrally to patient assessments and deployed automatically to all participants. This helps you reduce risk and study delays.
The Scribe App increases compliance and enhances the patient experience. It’s intuitively designed for Android or iOS using consistent features available online or off.
Whether with a provisioned device or bring your own device (BYOD), Scribe updates studies without requiring patients to download an updated app.

Discover how a large oncology sponsor created a customized eCOA library to collect accurate, daily reported patient data across 13 countries. There was no room for error to ensure each input could ladder up into one resolution.
IQVIA eCOA enabled the sponsor to create eight new COA instruments, manage 27 languages and obtain all the necessary validations and licenses to accelerate the successful outcome of this vital oncology trial.
Technology informed by unparalleled Decentralized Trials (DCT) operational experience and delivered at global scale.
Deliver eConsent to sites and study participants around the globe with confidence
Power your decentralized trial with an agile randomization and trial supply management solution that supports supply flexibility and optimization as well as adaptable direct to patient options.
Improve study quality, accelerate decision making and reduce site and sponsor burden by harnessing the power of IQVIA’s integrated Interactive Response Technology (IRT) and electronic Clinical Outcome Assessment (eCOA) solution.