
























- Blogs
- The Changing Role of Registries and Health Data Platforms
A session on registries and health data platforms during the IQVIA Institute’s Patient Advocacy Summit, held in December 2021, considered learning health system perspectives on the changing role of registries and data hubs in healthcare. This discussion, led by leaders from several patient advocacy organizations and IQVIA, examined areas of this rapidly changing landscape that will arm and empower advocacy organizations to develop leadership.
The Changing Data Landscape
The ways in which patient advocacy organizations are leveraging data are changing. There are five major drivers of change, requiring agility and enabling multiple value streams to be realized from data:
- Regulatory landscape (such as the 21st Century Cures Act and U.S. Food and Drug Administration (FDA) guidance on real-world evidence): This is leading to greater acceptance of real-world data/real-world evidence for post-market surveillance and other regulatory uses
- Technical environment (e.g., Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR) and other interchange standards, Observational Medical Outcomes Partnership (OMOP) and other common data models): This enables growth in the automation of data capture and data processing, and simplifies analytic product delivery
- Vendor ecosystem (e.g., cloud-based data stores and analytics pipelines, and a broader range of commercial partnering opportunities): This reduces the costs of delivery and simplifies scaling
- Expectations (e.g., patient centricity, data ownership, privacy, and security): These are driving a shifting focus to Patient Powered Research Networks (PPRNs) and patient communities
- Demand: There is an increasing need for agility and multiple value streams from data stewards; within healthcare, there is pressure to improve quality and reduce costs; and in the Life Sciences sector, there is a need to accelerate and de-risk drug development.
Traditional And Expanded Views Of Registry Use Cases
Traditionally, registries have been viewed as being useful in basic scientific research. Such uses included:
- Biomarker discovery
- Clinical research and surveillance, such as studies of natural history, effectiveness, and safety
- Healthcare quality improvement, such as quality improvement (QI) process measurement, QI outcomes measurement, guideline development, and guideline adherence
- Policy and advocacy
More recently, this view has been expanded and continues to evolve to include:
- Accelerating treatments and cures, for example, in clinical research networks for clinical trial recruitment, endophenotype discovery, and interventional research
- Product outcome evaluation to support regulatory requirements by providing real-world data and evidence
- Population health management, for example in chronic disease follow-up
- Patient care management and point-of-care support to inform clinical decisions and precision medicine approaches
- Patient self-care and direct-to-patient research engagement
Registries And Learning Health Systems
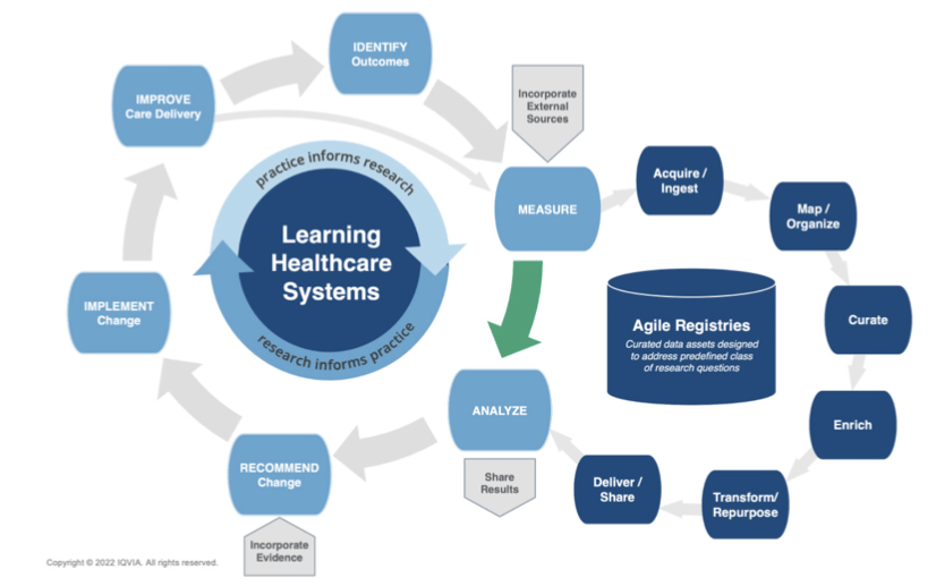
Learning health systems are ones where practice informs research and research informs practice. These employ a continuous loop to identify outcomes, measure, analyze, recommend and implement change, and improve care delivery (Figure 1). Within this cycle, there is friction in the move from measurement to analysis—the process known as ‘“data cleaning” or “data wrangling,” which can take 80-90% of all the effort required to answer an analytic question.
Agile registries can address this friction. Within such registries, curated data assets are designed to address a predefined class of research questions. Here, the data are acquired, mapped, curated, enriched, transformed or repurposed, and delivered back to the health system for analysis. This allows analytic data sets to be created in days as opposed to months or years.
Figure 1: The learning healthcare systems perspective: What are registries for?
The Role of Data, Registries and Patient Organizations
The increased power of patients to share their data under new U.S. legislation such as the 21st Century Cures Act is a major factor for advocacy organizations, providing access to information on a wider variety of patients, disease experiences and treatment journeys. The requirement for healthcare organizations to share patient data with advocacy organizations who have the patient’s consent is another key element.
An interesting trend in recent years has been the increasing strategic interest of pharmaceutical companies to partnering on registry programs, driven by investments in rare disease research and by advocacy organizations’ robust data collection efforts.
The new legislation is further enabling partnerships between companies and patient organizations in areas including the use of registry data as an external comparator in clinical trials. A major change is the need for more rapid release of data from registries to experts who can review and qualify the data, with the aim of identifying and filling data gaps at an earlier stage. This may drive registry owners to consider licensing the data for use before sending to regulators, enabling a more thorough review by potential partners at an earlier point.
The ways in which patient advocacy organizations are leveraging data are changing. In the U.S., changes are being driven by landmark regulatory advancements and an accelerating ability to access interoperable health data. These changes are fueling new approaches to data use and exciting opportunities for patient advocacy organizations.
Read the full whitepaper: “The Changing Role of Registries and Health Data Platforms”
